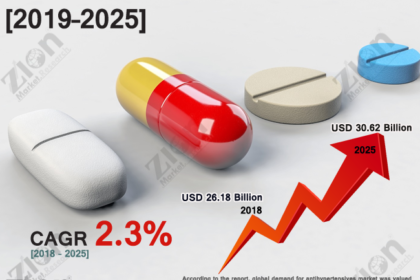
Plasma therapy denoted the procedure of substituting the plasma protein in patients experiencing deficiency of plasma protein. It widely finds application in androgenetic alopecia, face rejuvenation, and wound healing. With the rising requirement of minimally invasive and painless surgery methods, the plasma therapy market is projected to expand at a considerable rate during the forecast period. The other factors contributing to the global plasma therapy market growth include are growing incidence of life-threatening conditions, comprising numerous neurological system disorders like multifocal motor neuropathy (MMN), idiopathic thrombocytopenic purpura (ITP), and chronic inflammatory demyelinating polyneuropathy (CIPD). Further, the increase in the rate of contagious diseases like tetanus, rabies, and hepatitis A & B is also anticipated to drive the market growth.
In addition, with a striking technological progression over time has added much more to the therapies for treating numerous diseases that has, in turn, resulted in launch of commercial protein fractioning equipment from plasma. All of this expected to fuel the expansion of the global plasma therapy market in the years to come. And more recently, as Covid-19 has wreaked havoc globally, researchers are working to create antidotes for the novel coronavirus. Researchers and scientists are looking at numerous possibilities to arise with medical treatments that can battle the new coronavirus. And the one in focus at present is the convalescent plasma therapy. Many nations are actually looking at plasma therapy as a possible treatment for coronavirus. And this is projected to bring up several new opportunities for the global plasma therapy market in the coming period.
Get Sample of this Research Report for more Latest Insights – https://www.zionmarketresearch.com/requestbrochure/plasma-therapy-market
To cite, in late April 2020, approval has been granted by Food and Drug Administration (FDA), the US health regulator, to the Columbia University to initiate clinical trials to find out if blood plasma from survivors of COVID-19 can be utilized to inhibit infections within high-risk people. A $2.5 million grant has been made by Amazon to fund study at Columbia University Mailman School of Public Health.