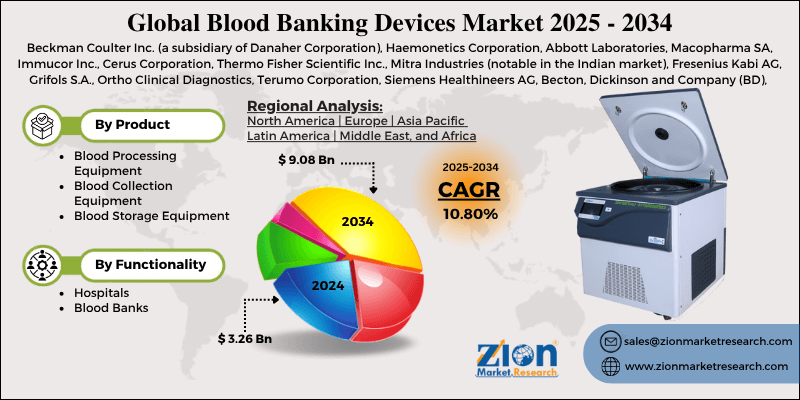
Blood banking devices are specialized equipment and instruments used in the collection, processing, testing, storage, and distribution of blood and blood components within blood banks, transfusion services, and hospital laboratories. These devices ensure the safety, quality, and availability of blood products—whole blood, red blood cells (RBCs), platelets, plasma, and cryoprecipitate—for transfusion medicine.
The field traces its roots to the early 20th century with Karl Landsteiner’s discovery of blood groups (1901) and the development of anticoagulation (citrate, 1914). Modern blood banking emerged post-World War II with plastic bags (1950s) and component separation. Automation accelerated in the 1980s-1990s with HIV screening mandates. As of 2025, the Global Blood Banking Devices market is valued at approximately USD 15-20 billion, growing at 5-7% CAGR due to rising transfusion demand (aging populations, trauma, oncology), stringent safety regulations, and automation/digitalization trends. Key players include Terumo BCT, Fresenius Kabi, Haemonetics, Grifols, Macopharma, and Beckman Coulter, with emphasis on pathogen reduction, apheresis, and cold chain management.
Blood banking devices uphold the “vein-to-vein” pipeline, ensuring donor blood becomes safe, efficacious products for patients.
Types of Blood Banking Devices
Devices span the blood product lifecycle:
- Blood Collection Devices
- Collection Bags/Systems: Plastic bags with anticoagulants (CPD, CPDA-1) and additives; single, double, triple, quadruple for component separation.
- Apheresis Machines: Automated collection of specific components (platelets, plasma, RBCs) while returning others (Trima Accel – Terumo, Amicus – Fresenius).
- Needles and Tubing Sets: Safety-engineered to reduce needlestick risk.
- Processing and Separation Devices
- Centrifuges: Refrigerated for RBC/platelet separation; high-speed for plasma.
- Component Extractors: Automated (e.g., Compomat G5) press bags to separate layers.
- Tube Sealers/Welders: Radiofrequency or heat sealers for sterile connections.
- Testing and Screening Devices
- Automated Immunoassays: For ABO/Rh typing, antibody screening, infectious markers (HIV, HCV, HBV, syphilis). Platforms: Ortho Vision, Immucor NEO.
- Nucleic Acid Testing (NAT) Systems: High-throughput PCR (Grifols Procleix, Roche cobas) for viral RNA/DNA.
- Blood Grouping Analyzers: Gel column or microplate.
- Pathogen Reduction Systems
- INTERCEPT (Cerus): Amotosalen + UV for platelets/plasma.
- Mirasol (Terumo): Riboflavin + UV.
- Reduces transfusion-transmitted infections.
- Storage and Preservation Devices
- Blood Bank Refrigerators: 1-6°C for RBCs; monitored alarms.
- Platelet Agitators/Incubators: 20-24°C with constant motion.
- Plasma Freezers: -30°C to -40°C; ultra-low (-80°C) for rare products.
- Cryoprecipitate Baths: Controlled thawing.
- Transportation and Monitoring
- Validated Shippers: Temperature-controlled boxes with data loggers.
- RFID/IoT Trackers: Real-time cold chain.
- Inventory and Data Management
- Blood Bank Information Systems: Barcode/RFID tracking, donor records.
Key Technologies and Innovations
- Automation: Robotic sample handling reduces errors.
- Pathogen Inactivation: Broad-spectrum safety.
- Extended Storage: Additives extend RBCs to 42 days.
- Point-of-Care: Bedside analyzers.
- Digital: Blockchain traceability, AI predictive inventory.
Regulatory Framework
- FDA (U.S.): Blood products as biologics; 21 CFR 600-680.
- AABB Standards: Accreditation.
- WHO: Essential for blood safety.
- EU: Directive 2002/98/EC.
Focus: Donor screening, testing, GMP.
Applications and Workflow
Typical vein-to-vein:
- Donor selection/screening.
- Collection (whole blood or apheresis).
- Testing/labeling.
- Processing (centrifugation, separation).
- Storage.
- Crossmatching/issue.
Devices ensure traceability, safety.
Market Trends
- Automation in developing regions.
- Pathogen-reduced products.
- Cold chain digitalization.
- Biologics (cell therapies) integration.
- Asia-Pacific growth (blood demand).
Challenges
- High cost.
- Blood shortages.
- Emerging pathogens.
- Supply chain disruptions.
Conclusion
Blood banking devices form the technological backbone of safe transfusion medicine, enabling efficient collection, processing, testing, and storage of life-saving products. Advances in automation, pathogen reduction, and digital monitoring enhance safety and availability amid rising demand. As regenerative medicine (e.g., CAR-T) intersects, these devices evolve to support complex biologics. Rigorous standards and innovation ensure blood banking remains a model of quality and patient safety worldwide.
More articles by ZMR Researche:
https://www.zionmarketresearch.com/de/report/refurbished-running-shoes-market
https://www.zionmarketresearch.com/de/report/one-wheel-electric-scooter-market
https://www.zionmarketresearch.com/de/report/industrial-adhesives-market
https://www.zionmarketresearch.com/de/report/sulphur-bentonite-market