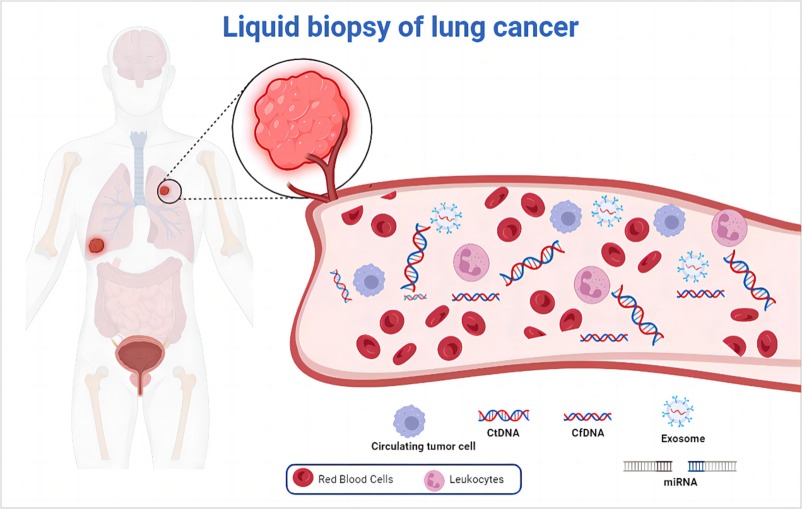
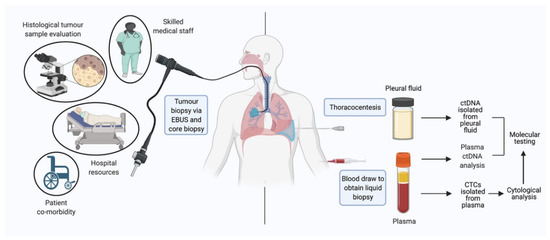
Lung Cancer Liquid Biopsy is a non-invasive diagnostic approach that detects and analyzes cancer-related biomarkers—such as circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), exosomes, or microRNAs—in blood or other body fluids to diagnose, monitor, and guide treatment for lung cancer. Unlike traditional tissue biopsy, which requires invasive procedures (bronchoscopy, needle biopsy), liquid biopsy offers a minimally invasive, repeatable method to capture real-time tumor dynamics.
The concept emerged in the late 20th century with early CTC detection, but exploded in the 2010s with next-generation sequencing (NGS) enabling ctDNA analysis. The first FDA-approved liquid biopsy test for lung cancer was the cobas EGFR Mutation Test v2 (Roche) in 2016 for EGFR mutations in non-small cell lung cancer (NSCLC). As of 2025, liquid biopsy is integral to precision oncology, with multiple companion diagnostics approved and a robust pipeline. The global lung cancer liquid biopsy market is valued at approximately USD 1-2 billion, growing rapidly due to rising NSCLC incidence (85-90% of lung cancers), targeted therapy adoption, and minimal residual disease (MRD) monitoring needs.
Biological Basis and Biomarkers
Lung Cancer Cells shed material into circulation:
- ctDNA: Tumor-derived fragmented DNA with somatic mutations (EGFR, KRAS, ALK, TP53).
- CTCs: Intact tumor cells; rarer but provide RNA/protein data.
- Exosomes: Vesicles carrying miRNA, proteins.
- Circulating RNA/miRNA: Regulatory molecules.
Key mutations in NSCLC:
- EGFR (10-35% adenocarcinoma, especially never-smokers/Asian populations).
- KRAS (25-30%).
- ALK rearrangements (3-7%).
- BRAF, MET, ROS1, RET (1-5% each).
Small cell lung cancer (SCLC) less mutation-driven; focuses on chemotherapy response.
Technologies and Methods
Liquid biopsy platforms vary by sensitivity and scope:
- PCR-Based
- qPCR/ddPCR: High sensitivity for known mutations (e.g., cobas, Therascreen).
- Limit: Single/few targets.
- Next-Generation Sequencing (NGS)
- Targeted panels (50-500 genes): Guardant360, FoundationOne Liquid, Resolution Bioscape.
- Whole-genome/exome: Broader but lower sensitivity for low-allele fractions.
- CTC Enrichment
- CellSearch (EPCAM-based), microfluidic (ISET, Vortex).
- Emerging
- Methylation/epigenetic profiling.
- Fragmentomics (ctDNA size/pattern).
- Multi-analyte (ctDNA + protein, e.g., Lunar-2).
Sensitivity: >90% for advanced disease; lower in early-stage/MRD.
Clinical Applications
- Initial Diagnosis Alternative when tissue inaccessible; detects actionable mutations.
- Treatment Selection Companion diagnostics for targeted therapies (osimertinib for EGFR T790M resistance).
- Monitoring Response ctDNA levels correlate with tumor burden; early drop predicts response.
- Resistance Detection Identifies emergence (e.g., EGFR C797S, MET amplification).
- Minimal Residual Disease (MRD) Post-surgery detection predicts recurrence.
- Screening (Investigational) Multi-cancer early detection (MCED) panels include lung; not yet standard.
Approved tests:
- Guardant360 CDx: Comprehensive NSCLC profiling.
- FoundationOne Liquid CDx.
Advantages
- Non-invasive, repeatable.
- Captures tumor heterogeneity (multiple sites).
- Faster results (days vs. weeks).
- Monitors dynamic changes.
- Lower risk than tissue biopsy.
Limitations
- Sensitivity in early-stage/low-burden disease.
- False negatives (shedding variability).
- False positives (clonal hematopoiesis).
- Cost (USD 3,000-6,000/test).
- Standardization gaps.
Guidelines and Adoption
- NCCN/ESMO: Recommend liquid biopsy if tissue insufficient or for resistance testing.
- FDA: Multiple companion diagnostics approved.
- Reimbursement: Expanding (U.S. Medicare for NSCLC).
Future Directions
- Improved sensitivity (ultradeep sequencing).
- MRD-guided adjuvant therapy.
- Screening integration.
- Multimodal (ctDNA + imaging).
- AI interpretation.
Conclusion
Lung cancer liquid biopsy has transformed precision oncology, enabling real-time, non-invasive tumor profiling for diagnosis, treatment selection, and monitoring. From single-mutation PCR to comprehensive NGS panels, it complements tissue biopsy while addressing heterogeneity and resistance. As sensitivity improves and costs decline, liquid biopsy will expand into early detection and routine surveillance, further personalizing care for the world’s leading cancer killer. Continued validation and standardization will solidify its role in guideline-directed management.
More articles by ZMR Researche:
https://www.zionmarketresearch.com/de/report/neopentyl-glycol-npg-market
https://www.zionmarketresearch.com/de/report/food-minerals-market
https://www.zionmarketresearch.com/de/report/optical-lens-for-infrared-device-market
https://www.zionmarketresearch.com/de/report/communications-service-providers-csp-market
https://www.zionmarketresearch.com/de/report/animal-radio-collar-market