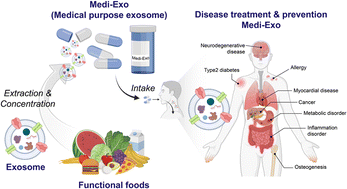
Rx Medical Foods, often referred to simply as medical foods, are specially formulated nutritional products intended for the dietary management of specific diseases or conditions with distinctive nutritional requirements that cannot be met by a normal diet alone. Despite the “Rx” prefix implying prescription-only status, medical foods in the U.S. are not required to be prescribed and cannot bear the “Rx only” symbol, as they are regulated as foods, not drugs.
They must be used under physician supervision, making them a unique category bridging nutrition and medicine. Medical foods play a vital role in managing chronic conditions, inborn errors of metabolism, and nutritional deficiencies associated with diseases. The global medical foods market is valued at approximately USD 24–26 billion in 2024–2025, projected to reach USD 33–40 billion by 2030–2032, growing at a CAGR of 5–6%, driven by rising chronic diseases, aging populations, and personalized nutrition trends.
History and Regulatory Framework
The concept originated in the mid-20th century with formulas for inborn errors of metabolism, like Lofenalac for phenylketonuria (PKU). Before 1972, they were regulated as drugs. The 1988 Orphan Drug Act Amendments defined medical foods, shifting them to food regulation.
In the U.S., the FDA defines them under 21 U.S.C. § 360ee(b)(3): Formulated for enteral consumption under physician supervision, addressing distinctive nutritional needs established by medical evaluation. No premarket approval required; exempt from nutrition labeling, health claims rules. Labels often state “use under medical supervision,” but no prescription needed.
Europe uses “Foods for Special Medical Purposes” (FSMPs) with stricter rules. Confusion arises as some products are marketed as “Rx” despite no legal requirement.
How Medical Foods Work and Key Characteristics
Medical foods provide targeted nutrients to manage disease-altered metabolism. For example, low-phenylalanine formulas for PKU prevent toxic buildup.
Criteria:
- Oral or enteral administration.
- For specific diseases with proven nutritional needs.
- Not for general conditions manageable by diet alone (e.g., not diabetes via regular low-sugar diet).
- GRAS ingredients or approved additives.
Examples of Medical Foods
Common products include:
- For Inborn Errors of Metabolism: Formulas like Phenex (phenylalanine-free) or Nepro for dialysis patients.
- Neurological/Psychiatric: Deplin (L-methylfolate for depression), VSL#3 (probiotic for IBS/ulcerative colitis).
- Pain/Inflammation: Limbrel (flavocoxid for osteoarthritis).
- Nutritionally Complete: Enteral formulas like Oxepa for critically ill.
Applications and Benefits
Used for PKU, renal disease, malnutrition, Alzheimer’s, and gastrointestinal disorders. Benefits: Targeted support, improved outcomes, reduced complications.
Advantages and Challenges
Advantages:
- No premarket approval speeds innovation.
- Physician-guided for safety.
- Potential insurance coverage in some cases.
Challenges:
- Regulatory ambiguity leads to misclassification.
- Variable reimbursement.
- Limited evidence for some products.
Market and Future Trends
Major players: Nestlé, Abbott, Danone, Fresenius Kabi. Growth from chronic diseases and personalized nutrition; emerging personalized formulas.
In summary, Rx Medical Foods offer vital nutritional management for specific conditions, distinct from drugs/supplements. Though not truly “prescription-only,” physician oversight is key. Consult healthcare providers for suitability.
More articles by ZMR Researche:
https://www.zionmarketresearch.com/de/report/gyrocopters-market
https://www.zionmarketresearch.com/de/report/gabion-boxes-market
https://www.zionmarketresearch.com/de/report/asset-optimization-solutions-market-size
https://www.zionmarketresearch.com/de/report/polyols-polyester-polyether-market
https://www.zionmarketresearch.com/de/report/hypersensitivity-pneumonitis-market